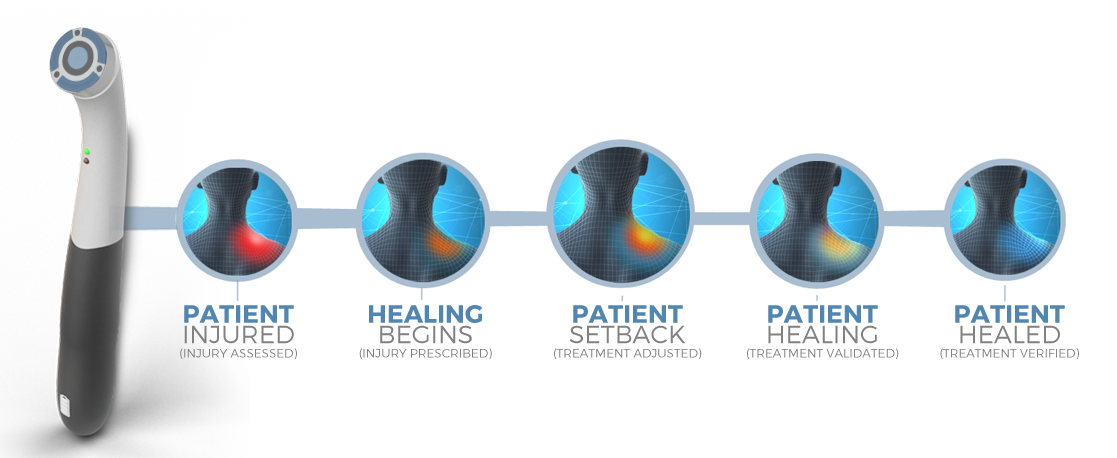
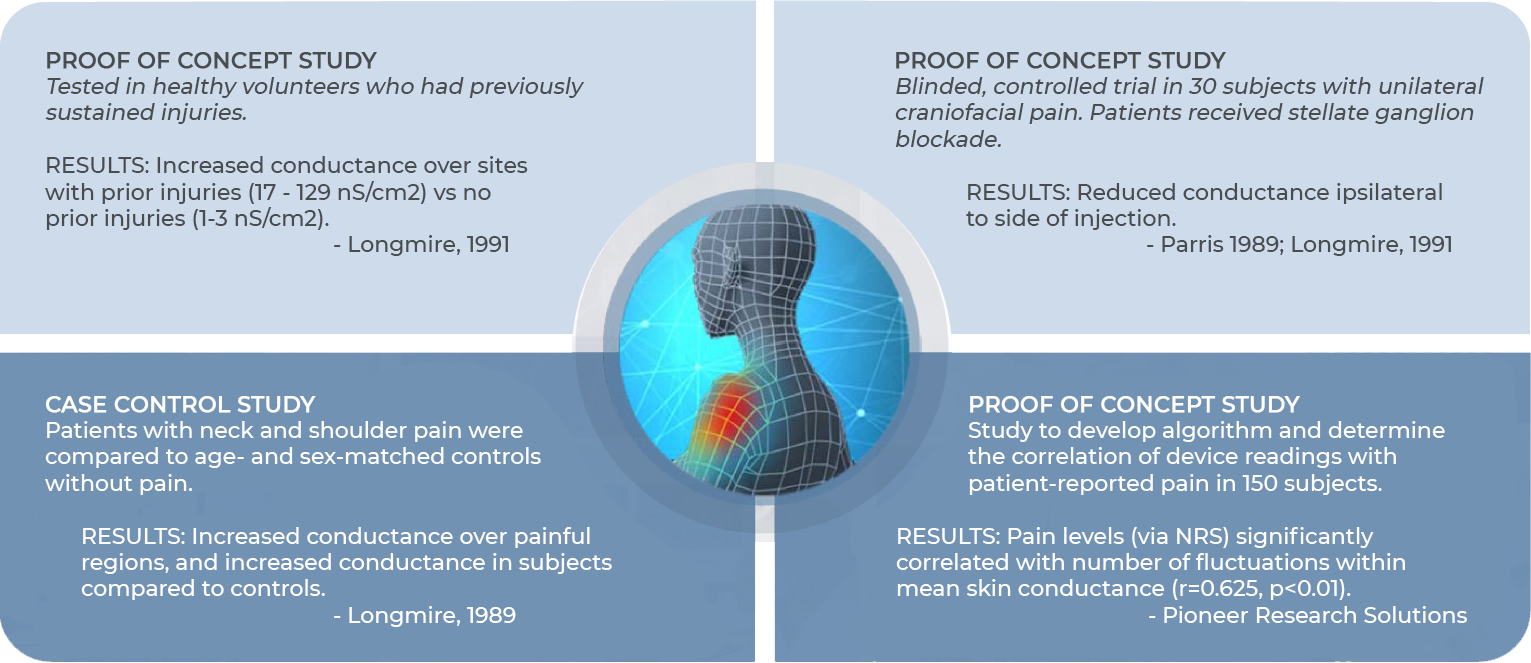
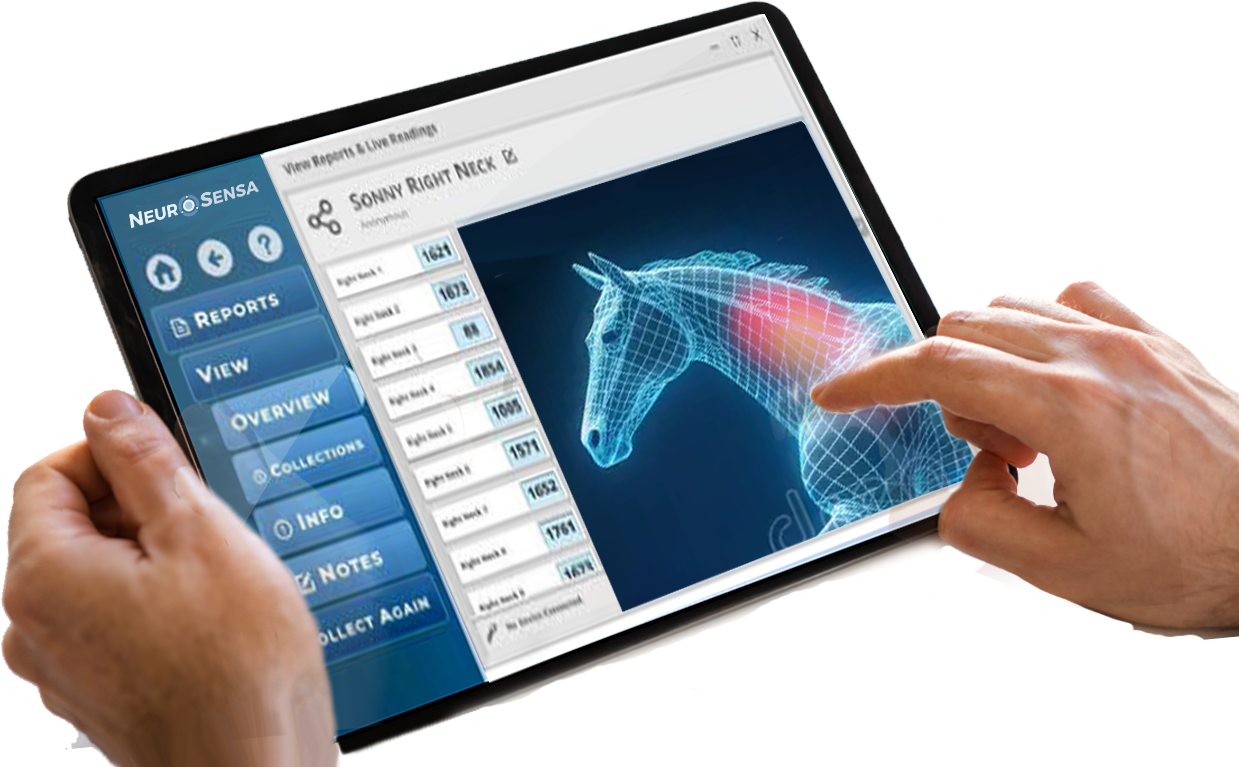
Case Studies and Validation
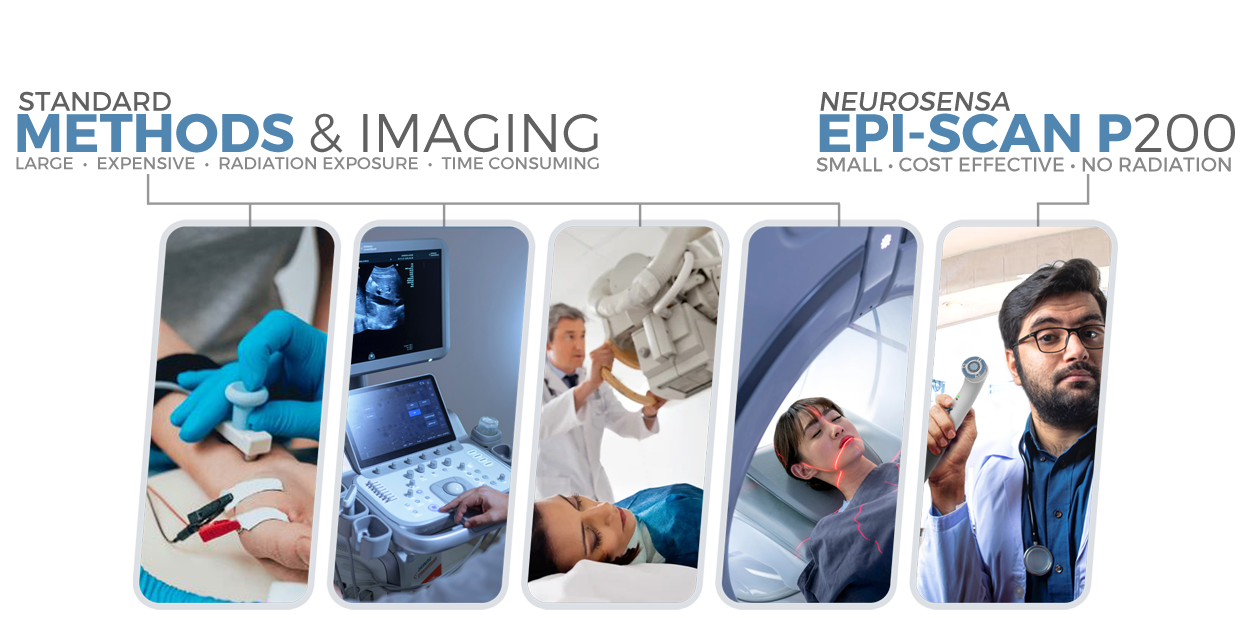
NeuroSensa has conducted several research studies to validate its pain analysis device. These studies used the device to measure skin conductance in patients with various pain conditions. Results consistently showed increased conductance in painful regions, correlating with patient-reported pain levels. For example, a case control study demonstrated heightened conductance in patients with neck and shoulder pain compared to healthy controls. A proof-of-concept study confirmed that fluctuations in skin conductance strongly correlated with self-reported pain levels. The device has been tested in different conditions, including nerve injuries and post-surgical pain, consistently providing objective evidence of pain through its real-time analysis of sympathetic nerve activity.
01 Validation Case
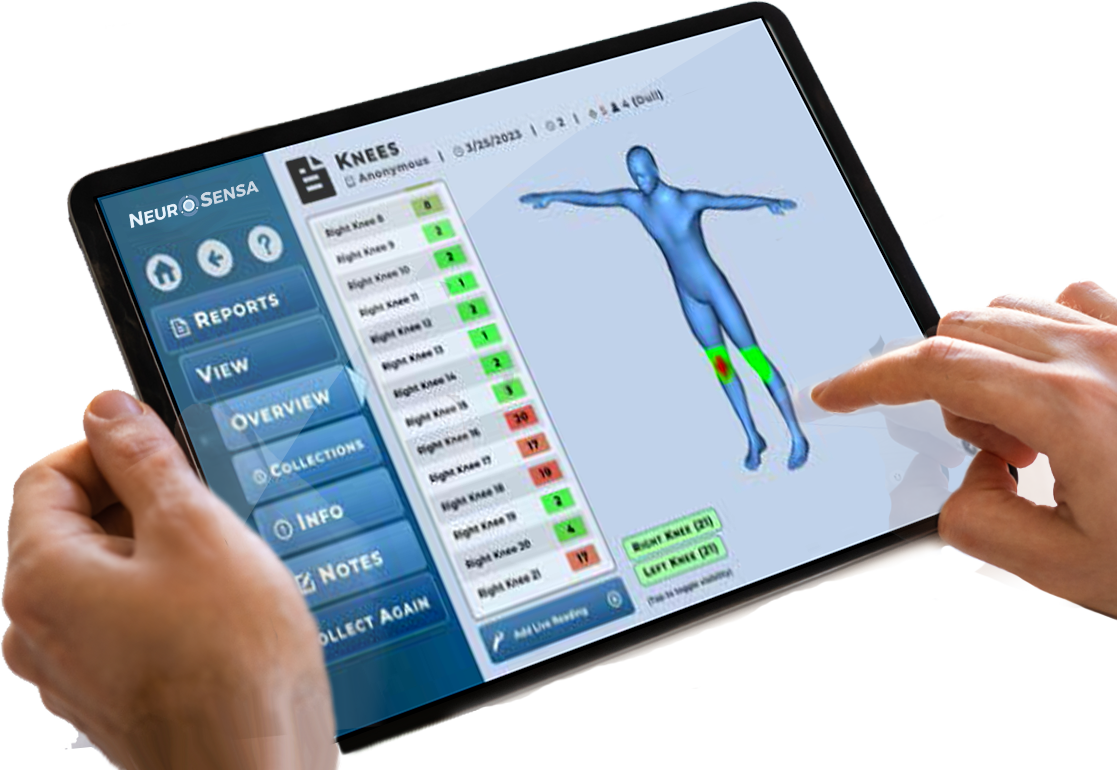
A 30-year-old female with a history of lumbar discectomy at L4-L5 experienced persistent pain following surgery, rated at 3/10 on the Numeric Rating Scale. Using the P-100 device and the NeuroSensa platform, increased activity was detected in her lower back, particularly around the spine and surgical scar. The visualization of her pain provided by the platform was a powerful moment for the patient, who expressed emotion and relief, stating, "I've never been able to visualize my pain like this." This objective assessment helped validate her pain experience and offered valuable insight for further treatment.
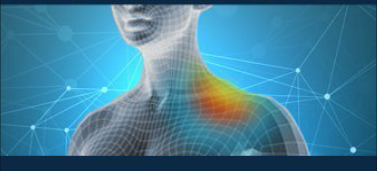
02 Confirmatory Case
A middle-aged female with a five-year history of right clavicle achiness and muscle spasms, following an injury from a golf ball, sought relief after several inconclusive X-rays and MRIs. Despite reporting a 0/10 pain level on the Numeric Rating Scale (NRS), she continued to experience discomfort. The NeuroSensa platform, using the P-100 device, detected increased activity over her right clavicle and neck muscles, confirming the presence of injury that other imaging methods had failed to identify. This objective validation helped to accurately pinpoint the source of her discomfort and guide further treatment.
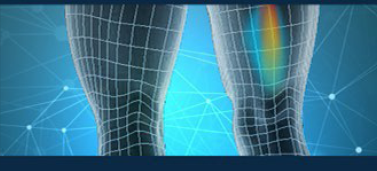
03 Legal Case
A patient with a saphenous nerve injury developed reflex sympathetic dystrophy (RSD), but Workers' Compensation disputed the diagnosis. Using the P-100 device and the NeuroSensa platform, objective evidence of RSD was provided, confirming the diagnosis. This data also supported the medical necessity for spinal cord stimulation as a treatment. As a result, the patient successfully won a six-figure settlement, thanks to the objective validation provided by the NeuroSensa platform.
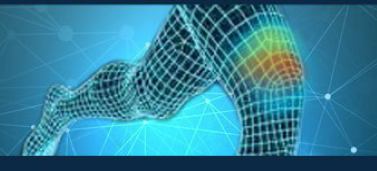
04 Actionable Insight
A male runner experienced pain on the side of his knee. Using the P-100 device and the NeuroSensa platform, it was revealed that the actual source of pain was above the knee. This actionable data led to a targeted treatment plan, including acupuncture focused on the area above the knee. As a result, the patient experienced significant relief and was able to run a marathon pain-free, an outcome that would not have been achieved without the precise insights provided by the device.
Trials and Pilot Studies
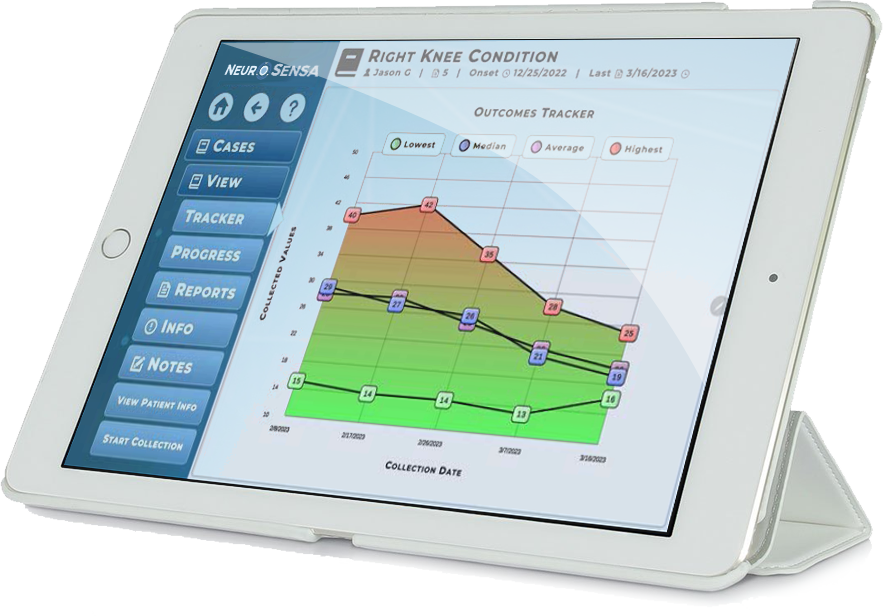
NeuroSensa has conducted several trials and pilot studies to validate its pain assessment technology. One case control study compared patients with neck and shoulder pain to healthy controls, showing increased skin conductance in painful areas. Additionally, proof of concept studies demonstrated significant correlations between device readings and patient-reported pain levels. For example, a study with 150 subjects confirmed the link between skin conductance fluctuations and pain, while a controlled trial in patients with craniofacial pain showed reduced conductance following targeted treatment. Another study revealed higher conductance in areas with prior injuries, further supporting the device’s ability to objectively measure and track pain.